Marbaloxavir (Xofluza) is a Cap-dependent endonuclease inhibitor and the only approved single-dose oral medication for the treatment of influenza. It is also the first antiviral flu drug with a novel mechanism of action to be approved by the U.S. FDA in nearly 20 years. It has been approved in multiple countries, including the United States, Japan, and China.
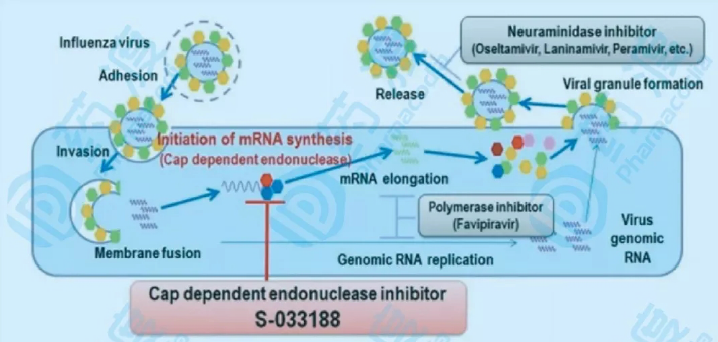
The LHZH developed by ChiHealBio Innovation Pharmaceutical Research Institute is based on the baloxavir molecule and has been specifically designed to combat viral resistance and enhance antiviral activity. It features broad-spectrum anti-influenza properties, exhibiting superior activity against both influenza A and B viruses, highly pathogenic avian influenza viruses, and more. It is expected to have an improved oral bioavailability, with no food effect, further enhancing its safety and efficacy. Additionally, it is not limited by the 48-hour window for administration, and remains effective even when taken up to 72 hours after the onset of symptoms. The drug is also less likely to develop resistance due to viral mutations, making it a hallmark of second-generation baloxavir.
Currently, the patents for other PA inhibitors under research or already on the market have long patent terms, making generic versions unlikely in the short term. The LHZH project developed by Nanjing Zhihe has already applied for a patent (Patent No.: 202111291506.9), and has independent intellectual property rights, free from restrictions imposed by related patents.

Baloxavir ethyl ester is a prodrug that is hydrolyzed into baloxavir in the body to exert its effects. Scientists at Peiyou have applied AI technology to conduct in-depth research on its structure-activity relationships. Using a combination of variational autoencoders and reinforcement learning with deep learning and machine learning, they performed target validation and activity screening. They further investigated the structural and physiological-biochemical characteristics of Cap-dependent endonuclease, recognizing, classifying, extracting, and analyzing foundational research data. This enabled them to accurately predict the "drug-target" interaction and optimize the design of candidate compounds with "me-better" potential.
Based on an in-depth study of the structure-activity relationships of benchmark compounds, they retained the crucial structural features necessary for anti-influenza activity. Additionally, they introduced two structural fragments that help counter viral mutations and enhance antiviral activity. As a result, the compound performed excellently in key non-clinical comparative studies, fully achieving the design goals: not limited by the 48-hour treatment window, less prone to resistance, and embodying the characteristics of second-generation baloxavir. To some extent, it can be considered an enhanced version of second-generation marbaloxavir.
Maturity and Risk Analysis
The HBJ strategy in the LHZH design approach has already resulted in a candidate drug entering Phase II clinical trials, and the research findings are promising. The strategy of introducing XI-related compounds has also received implied clinical approval. The parallel implementation of both strategies will not only significantly enhance the advantages of the candidate drug but also further ensure the likelihood of success, minimizing project risks and offering very high development prospects.
The LHZH compound in this project has formed its own patent while avoiding existing patents. Multiple discussions and simulations have been conducted with intellectual property experts, and a patent search has been commissioned with the Patent Search and Consultation Center of the National Intellectual Property Office. The search results show that no related published structures were detected, indicating that the compound meets the requirements for patentability in terms of novelty, inventiveness, and practicality. Multiple key studies on its in vitro and in vivo efficacy have shown significant effects.
At the same time, the synthesis process of LHZH reduces six steps compared to baloxavir, with fewer chiral centers. This will significantly lower production cycles and costs. Under the upcoming DRGs payment model, it will have a clear competitive advantage over other compounds with the same target that are either already on the market or under research.