Project Performance
In the past year, Beijing Chiheal Aikang Pharma, as a core force of the registration and clinical business segment of Chiheal Group, achieved significant results. In the registration business, we successfully completed the post-market change registrations for two imported drugs, obtaining the approval documents. Additionally, we timely completed the submissions for several in-progress products, including Pre-IND, IND, and NDA applications, which are now in the CDE review phase. The Clinical Medicine Department also achieved impressive results, completing the clinical data writing, communication, and medical consulting services for over ten in-progress products. Meanwhile, multiple clinical trial projects successfully met their research milestones, with results aligning with the expected outcomes, providing strong support for our clients to catch up with their submission schedules.


Honor and Certification
In addition, we successfully obtained recognition as a High-tech Enterprise in Beijing, a Technology-based Small and Medium-sized Enterprise, an Innovative Small and Medium-sized Enterprise, and received accolades under the Beijing Science and Technology Program for Expansion and Foundation Building. These certifications not only recognize our technical strength but also affirm our innovative capabilities.

Looking forward to the new year.
In the new year, we will embrace new challenges and opportunities with even greater enthusiasm and a stronger commitment. We believe that with the collective efforts of all our employees, Chiheal Aikang Pharma will achieve even greater success!

About Chiheal Aikang Pharm
Chiheal Aikang Pharma was jointly established by Nanjing Chihealbio and Beijing Aikang Pharma, and is a subsidiary controlled by Nanjing Chiheal bio. As an important part of the drug development chain of Chiheal Group, Chiheal Aikang Pharma serves both domestic and international pharmaceutical markets. It fully inherits and leverages Aikang Weijian’s business strengths in drug clinical trials and registration submissions. By relying on the natural alignment of the two companies' technological segments in the drug development service chain, Chiheal Aikang Pharma aims to establish itself as a top-tier clinical CRO service platform in China.
Core Business
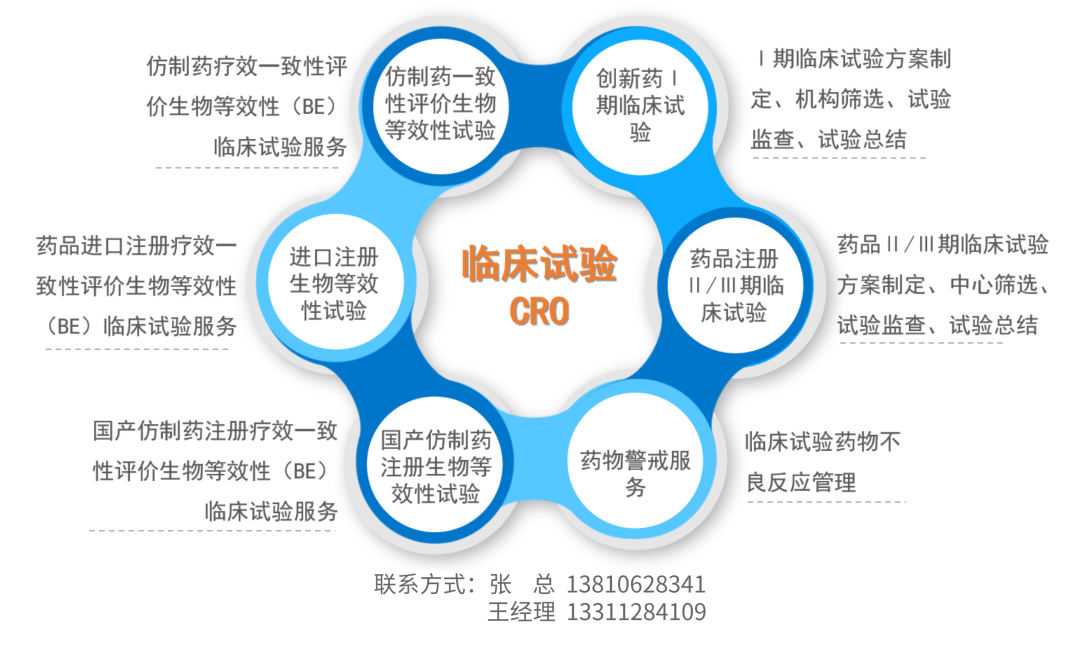
Clinical Research CRO Services
The specific content of clinical business includes clinical medical documentation design and writing, clinical/medical monitoring, third-party clinical auditing, clinical trials, and post-market pharmacovigilance services. The core clinical team leverages over 20 years of experience in the pharmaceutical development field, with notable strengths in clinical research in psychiatry, respiratory, anesthesiology, infectious diseases, and gastroenterology. Additionally, the team has extensive project experience in the clinical research of challenging drug metabolism studies, such as long-acting injectable formulations, endogenous substances, and inhalation drugs with high variability. The company provides professional CRO service support to domestic and international pharmaceutical companies, industry, and research institutions.
Registration and Filing Services
Provide drug registration and filing services, expert Q&A (consultation on various stages of registration and filing by experienced operational experts), and policy and regulation analysis (organizing, categorizing, and extracting applicable policies and regulations).