Nanjing Chiheal Biomedicine Technology Co., Ltd established in 2018 and located in Gulou district, Nanjing is a CXO enterprise founded by renowned industry experts, specializing in providing drug research and development technical services. Guided by the principles of being clinically driven, prioritizing innovative research, and centering on client needs, the company delivers premium and efficient one-stop comprehensive services. These include corporate development planning and market value management, product project evaluation and pipeline planning, pharmaceutical research, non-clinical study design and project management, clinical research, and regulatory submissions.
Through high-quality, high-efficiency, and cost-effective R&D services, the company supports clients in seizing opportunities presented by consistency evaluation, the MAH (Marketing Authorization Holder) policy, and centralized procurement initiatives. This approach empowers business development, enhances client enterprises, and enables all collaborating parties to achieve rapid and high-quality growth.

Chiheal Biomed, guided by the principle of "Innovation Drives Development," extends its commitment to global clients, offering opportunities for the transfer and collaborative development of impactful Fast-Follow First-in-Class (FIC) innovative drugs. We take pride in delivering a comprehensive, "one-stop" service for innovative drug development, encompassing drug design, synthesis, pharmaceutical development, and clinical research services. Our platforms are designed to cater to the diverse nee

We have a pipeline of novel, fast-follow PCCs (preclinical-candidate-compounds) for the therapies of metabolic and endocrine disorders.


We leverage a platform based on generic drug development and consistency evaluation services, highlighting our strengths in specialization and efficiency. Our expertise spans multiple dosage forms, including oral solid formulations, inhalation formulations, topical formulations, eye drops, and injections.


We have established robust technological platforms for fundamental and applied research in Traditional Chinese Medicine (TCM), encompassing theory, key technologies in innovative drugs, and critical technologies in the TCM industry.


We provide services including research project initiation, R&D strategy guidance, regulatory registration consultation, technical consulting, review of application documents, and mock inspections. To date, we have successfully completed dozens of technical service projects and guided multiple oral solid dosage forms through generic drug consistency evaluation studies.

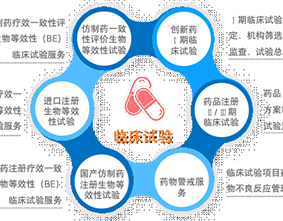
Aikang Pharma focuses its primary operations on Phase I-IV clinical trials and Bioequivalence (BE) studies, specializing in drug clinical research CRO services and comprehensive support for IND, ANDA, and NDA registrations. Our commitment lies in delivering pharmaceutical companies specialized services in drug clinical CRO and regulatory submissions for the Chinese market, expediting the process of product market entry.


Based on our proprietary pharmaceutical R&D platform, we independently initiate and develop projects. Upon achieving phased R&D results, we engage in various collaboration models.

Steadily advancing internationalization is a key component of our long-term development strategy. We consistently emphasize efficient and groundbreaking R&D innovation, balancing independent development with open collaboration. By integrating global resources and strengthening international partnerships, we build on our foundation of internal driving growth to achieve broader progress.
